What is the Role of Glutamic Acid Residue in Proteins?
The role of glutamic acid residue in proteins is a subject of great interest. Dr. Emily Zhang, a prominent biochemist, once stated, "Understanding glutamic acid residue can unlock new pathways in protein research." This highlights its importance in various biological processes.
glutamic acid residues are often found in the active sites of enzymes. They play a crucial role in catalysis and substrate binding. These residues can influence the protein's structure and function in unexpected ways. However, their complexity presents challenges. For instance, mutations at these sites can disrupt normal function, leading to diseases.
Researchers continue to explore how these residues interact with other molecules. The variability in their behavior raises questions. Can we predict their effects in every context? This uncertainty encourages further investigation. Understanding glutamic acid residue is essential for advancements in protein engineering and drug design.
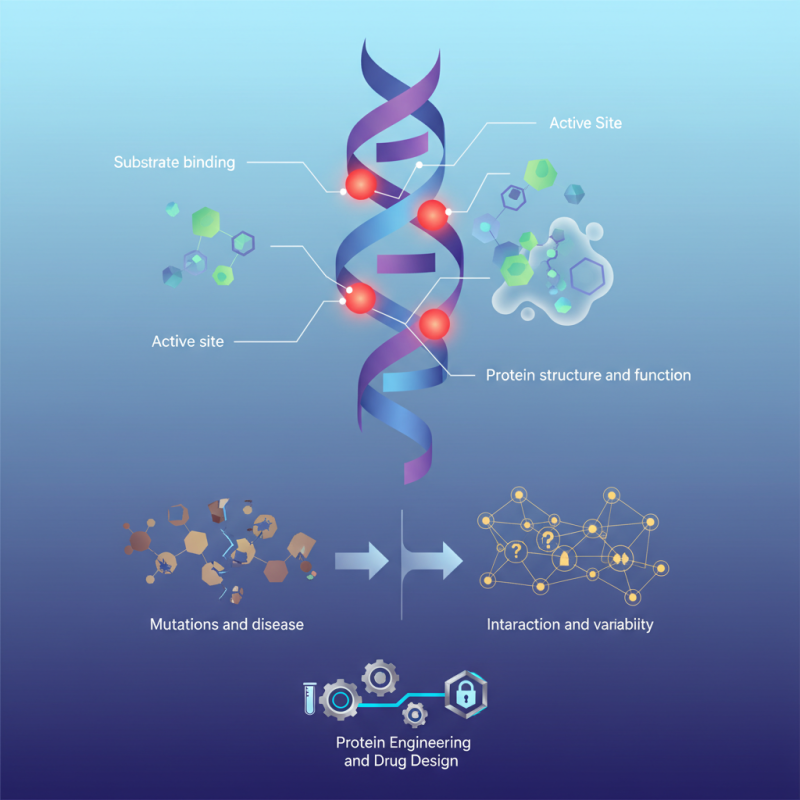
Overview of Glutamic Acid in Protein Structure
Glutamic acid is a vital amino acid in proteins. It plays a crucial role in maintaining the protein structure. Its side chain contains a carboxyl group that can attract and donate protons. This feature contributes to the stability and function of many proteins. Glutamic acid is not just about structure; it also affects protein folding and interactions.
When glutamic acid is present, it can form hydrogen bonds with nearby residues. This interaction helps stabilize the protein’s three-dimensional shape. It can also participate in ionic bonds, aiding in molecular recognition. However, its abundance can sometimes lead to destabilization if tightly packed in certain regions.
**Tips:** Always consider the environment around glutamic acid residues. Sometimes, small changes can impact protein behavior dramatically. Understanding these nuances enhances our grasp of protein dynamics. Balancing glutamic acid’s role is essential for optimal protein functionality. Watch out for instances where its presence may introduce challenges in maintaining structural integrity.
Role of Glutamic Acid Residue in Proteins
This chart illustrates the prevalence of Glutamic Acid (Glu) residues in various protein structures, highlighting their importance in maintaining protein stability and function.
Chemical Properties of Glutamic Acid Residues
Glutamic acid plays a vital role in protein structure and function. As a non-essential amino acid, it possesses unique chemical properties. The carboxyl side chain of glutamic acid carries a negative charge at physiological pH. This property contributes significantly to the protein's overall charge and folding. According to the Journal of Molecular Biology, the charge distribution in proteins can influence their interactions and stability.
The side chain of glutamic acid is also involved in hydrogen bonding and salt bridges. These interactions are crucial for maintaining the 3D structure of proteins. Research indicates that around 10% of all residues in proteins are glutamic acid. This concentration implies its importance in structural integrity. However, the abundance of glutamic acid can sometimes lead to regions of instability in proteins. High concentrations can disrupt normal interactions and cause misfolding.
In some cases, glutamic acid can participate in enzyme catalysis. Its acidic nature can facilitate the donation of protons in active sites. Data from enzymology studies show that mutations in glutamic acid residues often affect enzyme activity. Such findings highlight a potential area for further exploration. Understanding these chemical properties can enhance our grasp of protein functionalities and their roles in biological systems.
Functional Roles of Glutamic Acid in Enzyme Activity
Glutamic acid plays a crucial role in the function of many enzymes. Its side chain contains a carboxyl group, which is essential for enzyme catalysis. This feature allows the amino acid to participate in acid-base reactions. Oftentimes, glutamic acid acts as a proton donor or acceptor. Such interactions can stabilize enzyme-substrate complexes. Without this stabilization, reactions might proceed inefficiently.
Moreover, glutamic acid can influence protein structure. It is often found on the surface of proteins. Its hydrophilic nature can affect how proteins interact with water and other molecules. Sometimes, the positioning of glutamic acid is suboptimal. A poorly placed residue can disrupt enzyme activity and lead to decreased efficiency. Researchers are still exploring these complexities. They ponder how subtle changes affect protein function.
Another interesting aspect is the role of glutamic acid in signaling pathways. It can serve as a neurotransmitter in the brain, impacting neural communication. This duality is fascinating but also raises questions about its role in metabolic diseases. Understanding these dynamics is vital. It may lead to new insights into disorders linked to enzyme dysfunctions. Glutamic acid is indeed more than just an amino acid.
What is the Role of Glutamic Acid Residue in Proteins? - Functional Roles of Glutamic Acid in Enzyme Activity
| Function | Description | Significance in Enzyme Activity | Examples of Enzymes |
|---|---|---|---|
| Acid-Base Catalysis | Contributes to enzyme reaction by donating or accepting protons. | Facilitates the stabilization of transition states. | Chymotrypsin |
| Binding Interaction | Forms ionic bonds with positively charged residues. | Enhances specificity and affinity of enzyme-substrate interactions. | Carbonic Anhydrase |
| Regulatory Role | Involved in conformational changes affecting enzyme activity. | Plays a critical role in signal transduction pathways. | Phosphofructokinase |
| Cofactor Interaction | Acts as a site for cofactor binding which is essential for catalytic activity. | Necessary for the proper function of metalloenzymes. | DNA Polymerase |
Impact of Glutamic Acid on Protein Stability and Folding
Glutamic acid, an essential amino acid, plays a critical role in protein stability and folding. This negatively charged residue contributes to the formation of salt bridges and hydrogen bonds within the protein structure. These interactions can enhance stability, keeping the protein in its functional form. Interestingly, the presence of glutamic acid can also lead to misfolding. Sometimes, the charges may repel each other, causing improper interactions.
When proteins fold, the environment's pH can greatly affect glutamic acid's role. At different pH levels, it can gain or lose protons, altering its charge. This variation can influence protein dynamics significantly. For example, in acidic conditions, glutamic acid becomes more protonated, changing its interactions within the protein. It’s intriguing how a single change in pH may lead to different structural outcomes.
Despite its importance, glutamic acid may not always perform as expected. Proteins can accumulate misfolded structures if glutamic acid interactions fail. This challenge raises questions about how we can manipulate these residues for desired protein behavior. Understanding glutamic acid's role could lead to breakthroughs in biotechnology and medicine. Recognizing these terms reveals a complexity in protein chemistry that is both fascinating and, at times, frustrating.
Associations of Glutamic Acid with Disease Mechanisms in Proteins
Glutamic acid, an amino acid, plays a vital role in protein structure and function. It is known for its negative charge, which influences protein interactions and stability. In various diseases, glutamic acid residues can be found at critical sites within proteins. These residues could disrupt normal functions, ultimately contributing to disease progression.
For instance, in neurodegenerative diseases, glutamic acid can form toxic aggregates. These aggregates accumulate and lead to cellular stress. It’s fascinating to observe how one small change in amino acid composition can have such large implications. Researchers are uncovering how these alterations in glutamic acid impact pathways related to Alzheimer's and Parkinson's diseases, but many questions remain unanswered.
Moreover, the role of glutamic acid in enzymatic activity is equally intriguing. Mutations that alter glutamic acid residues can hinder enzyme function. This impacts metabolic pathways and causes a chain reaction of issues. Understanding these links can be complex. While some data show promising correlations, our knowledge is still incomplete. It raises the question: How can we use this understanding to develop targeted therapies?
